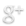The intensive prostate needle biopsy processing discussions, which occur periodically on HistoNet, convinced me to summarize the pre-analytical stage of biopsy handling in the histology laboratory. This post tries to have a comprehensive approach to the procedure with concentration on details without any attempt to have a scholarly study.
General approach to prostate needle biopsy grossing technology
Three main aspects of prostate needle biopsy grossing technology can be defined: specimen’s identification, completeness of submission, and appropriate representation on the block after embedding (Diagram 1.) The specimen’s identification is not only in the misidentification prevention that includes the patient/specimen requisites, but the identification of the corresponding sextant core/s location and other, if applicable, data relevant to the pathologist’s diagnosis data. Completeness of submission assures that all harvested material will appear on the slide. Representation on the block should reflect alignment, regularity of embedding, and most importantly, uniformity; that implies the same plane position of all cores on the surface of the block for section. Sampling lays the ground for correct embedding.
Diagram 1. Main aspects of prostate needle biopsy grossing technology
Transfer from the submission container into the tissue processing cassette.
There is a consensus that not more than two cores should be in one processing cassette to keep all parts of the cores flat at the same level. For example, the pathology department at John Hopkins Hospital recommends: “submitting one or two cores per container, which might reduce fragmentation and thereby improve the ability to diagnosis, quantify, and grade cancer in needle core tissue”. Three cores in the cassette is acceptable, but not more.10 In saturation procedure, which is not common practice, different rules are applied.
As an accepted technique, the cores are placed between two foam polyester sponges to keep the core/s flat and prevent losing small fragments, although the latter is questionable. The use of foam pads is methodologically controversial.
There are suggestions to use special cassettes for prostate needle biopsies. For example, Vortex™ Micro Cassette has an oval concave cavity with no corners that is convenient for small biopsies. This cassette, however, does not solve the problem of flattening the core as keeping it between foam pads does.
Details of completeness of submission and flattening the core/s will be presented in the pending publication in Journal of Histotechnology.
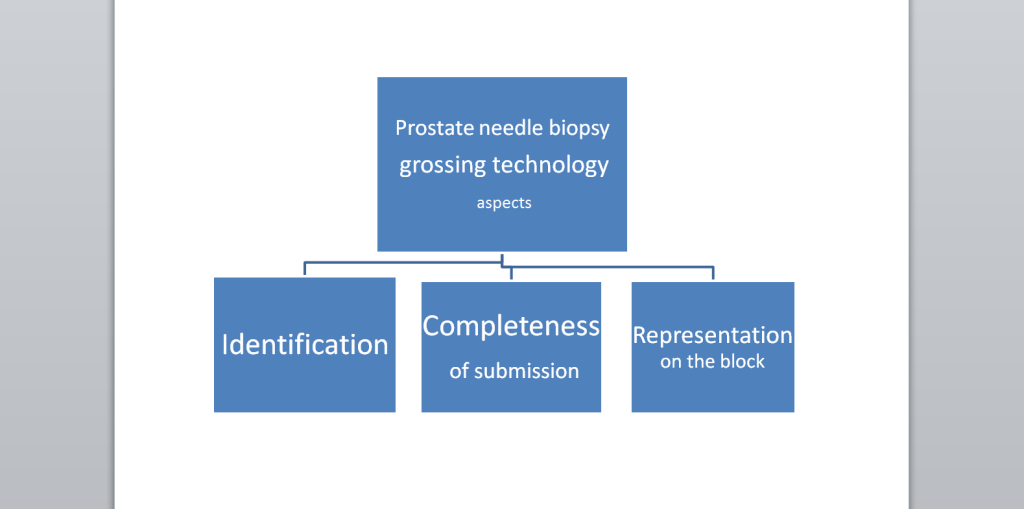
Representation on the block
Diagram 2. Representation of the prostate needle biopsy on the block
The prostate needle biopsy core’s representation on the slide is obviously determined by the embedding block. Professional grossing technology always bears in mind how the sample will be embedded in the block.
Tissue tampers with a short handle(Sakura’s Accu-Edge Grossing Tools)1 and a relatively convenient gadget HistoPress can be used for flattening during embedding
Sampling identification
Specimen misidentifications do occur, but it is sometimes exaggerated to the extreme. For example, some urology groups are demanding to send-out every “Positive” (with cancer) prostate biopsy for the “Know Error” DNA identity test.16 The test is used to confirm the identity of the patient diagnosed with cancer by matching the DNA to that obtained from a buccal swab from the patient. Besides the cost of the procedure, collecting a buccal smear adds even more variables. Perhaps, in the future, sending out DNA testing on all specimens, not just prostate biopsies, will be a standard. For now, however, this practice would only submit unjustified doubts in the pathology diagnosis.
Prostate needle biopsy sampling identification includes the standard specimen misidentification protocols, as well as some specifics. In the standard protocols, such as bar-coding confirmation by scanning labels on the container and other necessary procedures, it should also be included in the recommendation to follow as much as possible the rule of avoiding processing sequentially similar tissue specimens. It is desirable during accession, but especially important while sampling prostate needle biopsies. Of course, this is practically impossible in “monoculture” specialized laboratories.
The experience of a laboratory, which receives urology biopsy specimens, is interesting as an attempt to prevent patient specimen identification errors by inking prostate cores from all vials for a particular patient. The cores are inked by the grossing technician with the same color. Five colors are used sequentially: green, blue, yellow, orange, and black. The embedding histotechnologist checks each core to verify that it is the same color as all other cores for the particular patient. The pathologist reading the microscopic slides compares the ink color staining the tissue microscopically with the ink color dictated by the grossing technician.The trend to distinguish prostate needle biopsies from different patients by different color ink is stretching to even putting some colored glass bids in the cassette and after processing mark the same colored pencil lines the top of the slides during sectioning.
Conclusion
The trend of bringing sampling of a prostate needle biopsy in accordance with the pathologist’s diagnostic needs will continue. A comprehensive approach to grossing prostate needle biopsy grossing technology presented in this summarization can be useful for understanding details of pre-analytical processing and might be the background for further development of sampling techniques.