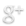Polyester blue rectangle foam pads (of course also different colors and shape) are ubiquitous in histology laboratories. They are called also sponges. The pads are used not only in North America, but in Europe, including Russia. Nevertheless, there are some controversies that accompany their use.
Technical
There is a notion among histotechnologists that microwave-assisted equipment manufacturers oppose the use of the foam pads. Laboratories had warranty issues (Sakura’s Finetek Xpress), that led to discontinue using the pads.
Sponge pads have a side of concern in short term processing, especially in microwave assisted method. It is the carryover from solution to solution possibility. Now the manufacturers (Sakura, Milestone) softened their objections to the pads. However, as a practical measure of making sure that the air is removed from the sponge to facilitate exchange, presoaking the pads in formalin should be obligatory. Some laboratories use alcohol formalin, as way of preventing additional exposure to formaldehyde, because for practical reasons, the open jar with presoaked pads is sitting on the grossing table.
Inquiries that were made could not confirm this. For example, Milestone’s international applications manager Jim Milios stated in personal communication that this notion is not accurate. The pads indeed bring some uncertainty in transfer fluids in chambers for any tissue processors type and the timing of the process because the latter is calculated by average size of specimens. However, if the presence of pads is taken into calculations of the cycle’s length during initial implementation of the program, these problems can be solved. When the pads are only minimally applied in the batch, it does not matter. When they compose the main part of the batch, adjustments should be applied. However, this applies to laboratories, which are solely dedicated to certain types of biopsies, for example prostate. In most laboratories the optimal tissue processing protocol is prepared that applies to any type of tissue.
Methodological
I am the enthusiastic proponent of sponges in surgical pathology grossing. On my grossing tray is always a jar with sponges for different purposes, from applying mordant on inked specimens to specimen orientation, especially in dermatopathology. This website includes the article “Biopsy Orientation in the Processing Cassette”, where pads are used for pre-embedding orientation.
However, the pads are a net of holes with sharp plastic edges. It may be suspicious that, under modern vacuum and pressure tissue processing conditions, the pads might be a trap for small fragile parts of the biopsy. Sometimes, it is called as compression artifact. They should not be used for orientation, for example, of a villous colon polyp or bladder biopsy. Definitely, they should not be used in a case of minuscule or fragile, as well as mucinous specimens.
The holes are, by size, comparable with thin parts of the prostate biopsy 24 gauge needle cores. Although usually a 18 gauge needle is used, some areas of cores, especially at their edges, are thinner. Under conditions of vacuum and pressure processing, these parts can be trapped in the holes. Those fragile parts might be valuable diagnostic areas of the biopsy in opposition to the stromal part of the core.
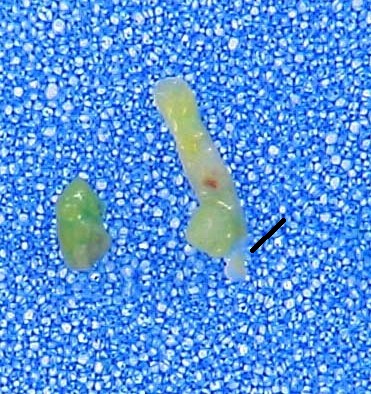
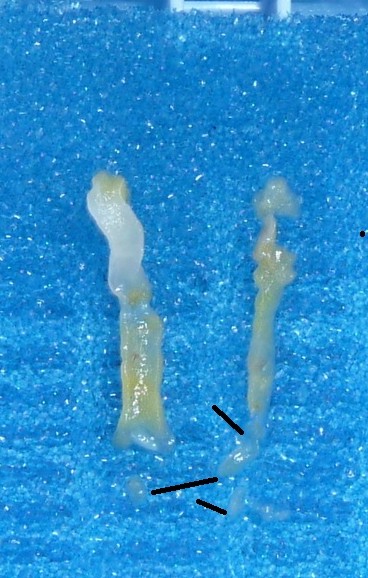
I conducted a “minor study” on this issue at the surgical pathology laboratory. Two randomly chosen cases of prostate needle biopsy taken by an experienced urologist in an academic institution might support the considerations presented above. I simply placed the cores on blue polyester pads and took regular focused pictures. The holes in the pad net are comparable with many areas of the cores, meaning that the most fragile acinar structures might be trapped in them by vacuum and pressure. On the picture below, the narrowing is pointed by arrows, the holes are pointed by straight lines.
It would be reasonable to perform a study to examine such pads microscopically after processing. However, there are substantial technical and patient safety obstacles. First, from bioethical point, the patient’s diagnosis should not be jeopardized using methodology that the professional considers wrong, even if it is widespread. Second, it is technically difficult to perform microtomy with the polyester pad because it is necessary to bring it to sectionability comparable to paraffin or other appropriate soft tissue media, such as after bone decalcification. Otherwise, the soft tissue of interest would not be presented adequately on the slide. In short, such a study requires some experimentation with questionable result. Perhaps, somebody will pick up this task. At this point in my opinion, would it not be right to abandon all direct contact between the cores and the pad during processing, without waiting for this study?
Already mentioned Jim Milios observed a method, when a single layer sheet of permeating paper is placed on a sponge to prevent absorption of core fragments into sponge creating a “sandwich” –sponge –paper-core-paper-sponge.
It is obvious, that pre-soaking or moisturizing the pads would be obligatory, if the specimen were placed directly on the sponge. If the paper protection is used, it is optional, but it is easier to position the specimen onto the wet sponge in the cassette.
Both mentioned case are presented entirely in a separated post Prostate Needle Biopsy and the Pad. It was impossible to publish them in a scientific journal’s article due to space limits and reviewers objection, but they demonstrate that the mentioned comparability of many cores’s areas with the size of the net’s holes is not an exception. The arrows are pointed on the cores, the lines are pointed on the net’s holes. The pictures look overcrowded, but informative and perhaps impressive.
Technical details of using polyester sponge pads for sampling prostate needle biopsy were sent for pending publication in Journal of Histotechnology.
Just as a parenthetical remark regarding the use of pads in core biopsies, I include some observations about breast core biopsy grossing technology. Although the gauge 14 thickness of the core is incomparable with the pad’s holes, I would not use them in breast cores either. The cores also are sometimes fragmented. Would they perhaps get trapped in the pad’s holes? In this regard, I would be concerned about small fragment with very thin attachment to the main core (see lines).